Most asthma interventions that were being implemented at the time of this early NCICAS research were either approaches to improve medication use, or general, “one-size-fits-all”, educational lectures or handouts. The NCICAS investigators wanted to design an approach that addressed the unique combination of risk factors that existed in this population. More than one thousand families (1,033) were enrolled in the NCICAS intervention and were randomized to a tailored Asthma Counselor intervention, or usual care. To achieve the custom and tailored intervention, a comprehensive computerized risk assessment tool, called the CARAT (Child Asthma Risk Assessment Tool) was designed. The CARAT consisted of 36 questions designed to assess each of the risk domains identified in NCICAS Phase I.
Based on the answers to these questions, and the skin test allergy results for the child, a computerized tailored report was automatically generated. This report could be as short as a few pages, or longer than 40 pages – each CARAT report was unique to the child and family. The CARAT report also included handouts (in English or 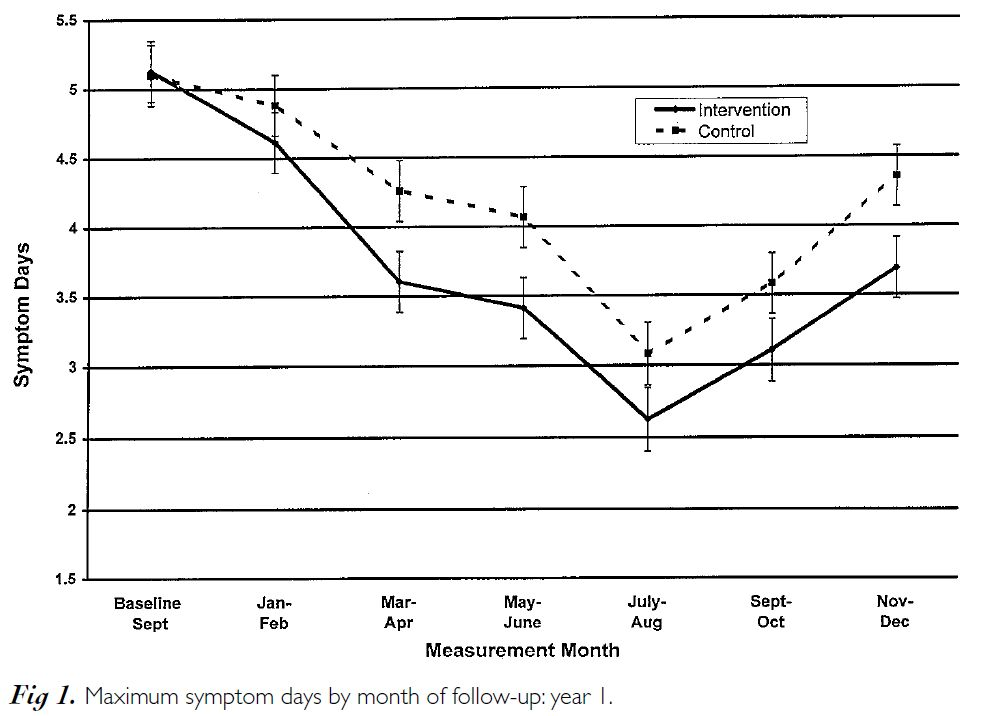 Spanish) that could be given to the family for the child-relevant risks, as well as background information for the Asthma Counselor to facilitate their discussions with the family. Note the graphical risk profile on page 8, followed by handouts for the family that follow. These handouts are provided in 3 formats; first, with mostly pictures to address low-literacy; secondly, with more text explanation for the family; and finally, in considerable detail for the Asthma Counselor.
Spanish) that could be given to the family for the child-relevant risks, as well as background information for the Asthma Counselor to facilitate their discussions with the family. Note the graphical risk profile on page 8, followed by handouts for the family that follow. These handouts are provided in 3 formats; first, with mostly pictures to address low-literacy; secondly, with more text explanation for the family; and finally, in considerable detail for the Asthma Counselor.
The environmental risks were linked to the child’s specific skin test allergy results to ensure that interventions were undertaken only when the environmental risk was appropriate for the child’s sensitivities and exposure combinations – otherwise, a brief educational discussion was conducted for those environmental risks that were not of immediate importance.
This semi-automated, highly tailored report and intervention led to a significant reduction in the child’s symptoms during the course of the year and was especially effective for those with the most severe asthma.
The symptom reduction continued through the second year, even though the intervention had ended [1]. In addition, this intervention was found to be very cost effective [2].
Footnotes
[1] - Evans, R., Gergen, P., Mitchell, H., Kattan, M., Kercsmar, C., Crain, E., Anderson, J., Eggleston, Malveaux, F., Wedner, J. A randomized clinical trial to reduce asthma morbidity among inner-city children: Results of the National Cooperative Inner-City Asthma Study (NCICAS). Journal of Pediatrics, 1999, 135(3) 332-38.
[2] - Sullivan, S.D., Weiss, K.B., Lynn, H., Mitchell, Kattan, M., Gergen, Evans, R. The Cost-Effectiveness of an Inner City Asthma Intervention in Children, Journal of Allergy and Clinical Immunology, 2002;110:576-81.